Silver
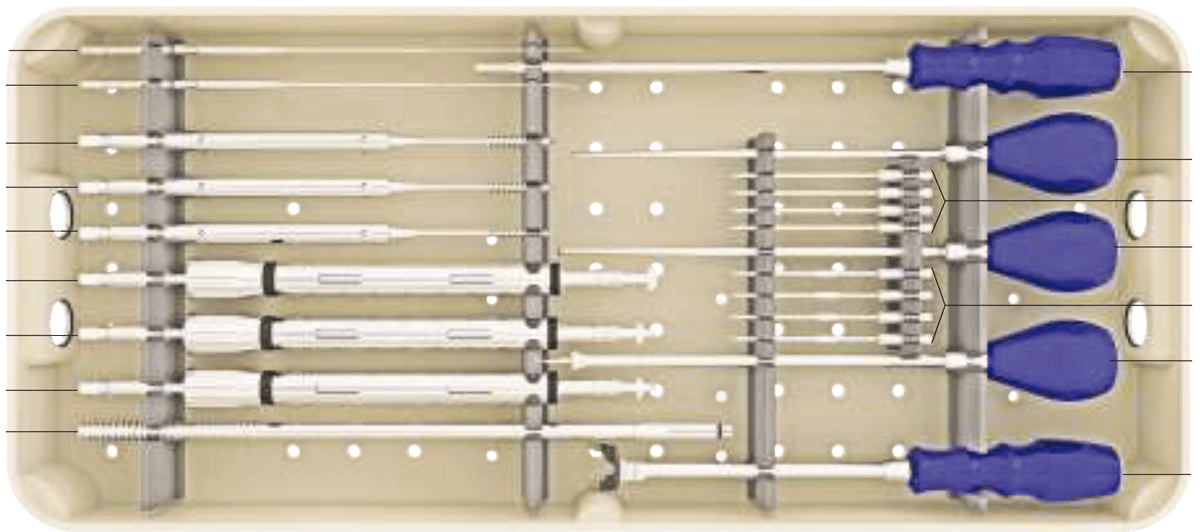
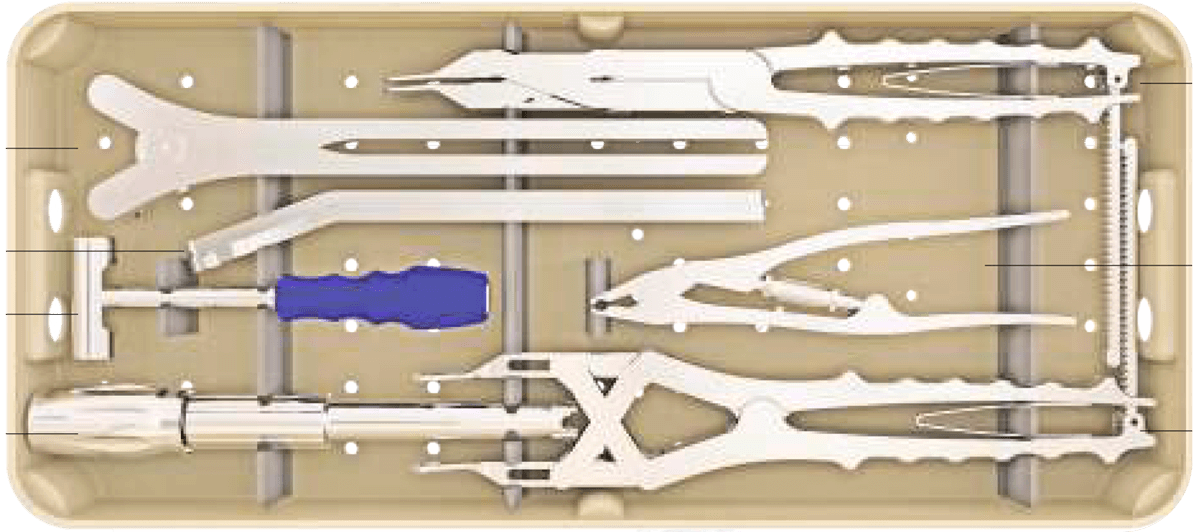
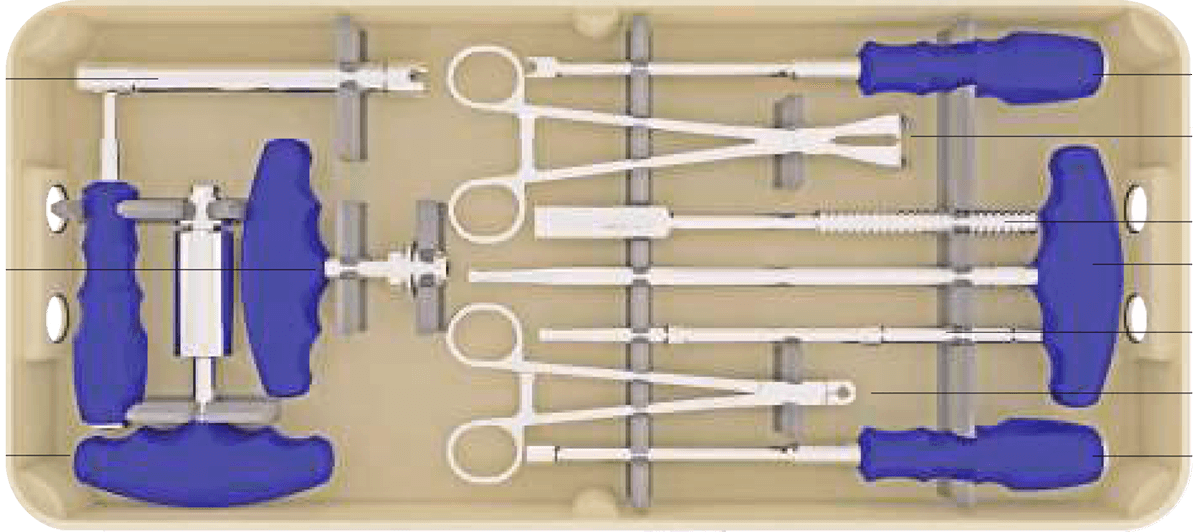
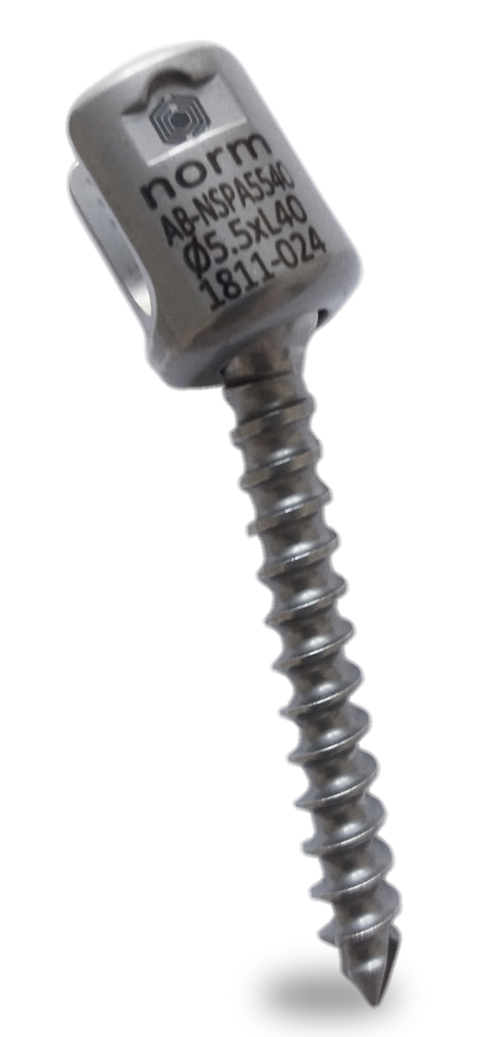
Sistema de instrumentación pedicular toraco-lumbo-sacro-pélvico de titanio con recubrimiento de plata antibacteriano.
Recubrimiento de plata antibacteriano Silver
Reducción del riesgo de infección aguda y subaguda.
Implantes esterilizados individualmente.
Disminución del tiempo de hospitalización.
Compatibilidad con RX, TAC, RNM, GGO, PET y SPECT
Tecnología única
El sistema espinal recubierto de nanopartículas de plata es una tecnología única, que por sus características antibacterianas reduce la tasa y el riesgo de infección aguda y subaguda en intervenciones de columna.
Reducción de las tasas de infección
La duración de las operaciones de estabilización o fusión de columna mediante sistema de tornillos pediculares y barras, que es uno de los procedimientos rutinarios en cirugía de columna, los factores predisponentes a la infección como las grandes incisiones, y la implementación de materiales (tornillos de aleación de titanio), conllevan un riesgo aumentado de infección.
En el caso de que la misma se produzca, las antibioterapias endovenosas y el aumento de los días de ingreso hospitalario, incrementan grandemente el coste del tratamiento. Adicionalmente, los antibióticos de que disponemos no siempre son suficientes para solucionar el problema, y en algunos casos, la retirada del material, lo que conlleva una segunda intervención, es necesario.
Esto afectará negativamente la calidad de vida del paciente, el tiempo de hospitalización, el coste del tratamiento, y la confianza del paciente tanto en el cirujano como en el centro hospitalario. La bibliografía reciente sostiene que los implantes de titanio recubiertos de nanopartículas de plata que se han implementado en cirugías de alto riesgo de infección en Traumatología y Ortopedia reducen la tasa de infección. Los centros con más experiencia en este tipo de implantes son el Royal Orthopaedic Hospital Oncology Service, Royal Orthopaedic Hospital NH Foundation Trust (Birmingham, UK), y el Department of Orthopedics and Tumor Orthopedics, Münster University Hospital (Münster, Germany), que desde 2015 publican los resultados de sus megaprotesis tumorales recubiertas de nanoparticulas de plata¹ ² ³.
Certificaciones
El Sistema Silver de instrumentación toraco-lumbo-sacro-pélvica cuenta con las certificaciones CE, ISO 9001, ISO13485, FDA y directiva de dispositivos médicos 93/42/CEE.
Los implantes de plata
Actualmente, la plata está ampliamente utilizada en el campo de los implantes de odontología y a grandes concentraciones, como cauterizador.
La plata es un material algodinámico, es decir, que permite la emisión espontánea de iones que están en su superficie, y estos iones son antibacterianos. El cobre, el zinc y el oro son también materiales algodinámicos, pero por su coste y por algunas características tóxicas, no tienen las ventajas de la plata.
Están ampliamente estudiadas las características bactericidas y bacteriostáticas de la plata⁴ ⁵ ⁶ sobre los microorganismos eucariotas (bacterias y hongos) por diferentes mecanismos: produce enzimas que evitan la mitosis, anulando la replicación del DNA bacteriano; eliminando la impermeabilidad de las membranas celulares produciendo así una insuflación del microorganismo y la consecuente apoptosis del mismo; alterando el metabolismo celular ocasionando la necrosis del germen.
Los implantes del Sistema Silver están fabricados en aleación de Titanio (Ti6AL4V) + recubrimiento con nanopartículas de plata por la ruta sol-gel a partir de nitrato de plata⁷ ⁸ ⁹. Está testado que el sistema espinal de titanio recubierto de plata mediante el método de la nanotecnología tiene un efecto antibacteriano eficaz manteniendo las características físicas y biomecánicas del material *.
El volumen de recubrimiento de plata en los implantes se realiza en un grosor de micras, y en un peso de microgramos por biocompatibilidad *. Una vez instrumentado un paciente un paciente con implantes con recubrimiento de plata, se producen unos aumentos del nivel de plata en torrente sanguíneo hasta un máximo de 5 mg. El cuerpo humano en su estado normal posee aproximadamente 1 mg de plata. La menor cantidad de plata documentada que ha producido argiria es de 4-5 gramos. Así, los niveles máximos de plata en sangre adquiridos post implementación de implantes recubiertos de nanopartículas de plata (máximo 6 mg) ni se acercan a los niveles tóxicos que causarían argiria aguda o crónica.¹⁰ ¹¹
Los productos se sirven estériles por el método de óxido de etileno. Si el producto, por cualquier motivo se desesteriliza, se puede reesterilizar en autoclave (134°C 5 min). No se deberían utilizar si existe alguna duda sobre la esterilidad del producto.
Los implantes son compatibles con RX, TAC, RNM, GGO, PET y SPECT.
Evidencia científica de implantes de titanio recubiertos de plata
Otros artículos:
*SILVER Biomechanical Data & Biocompatibility Report